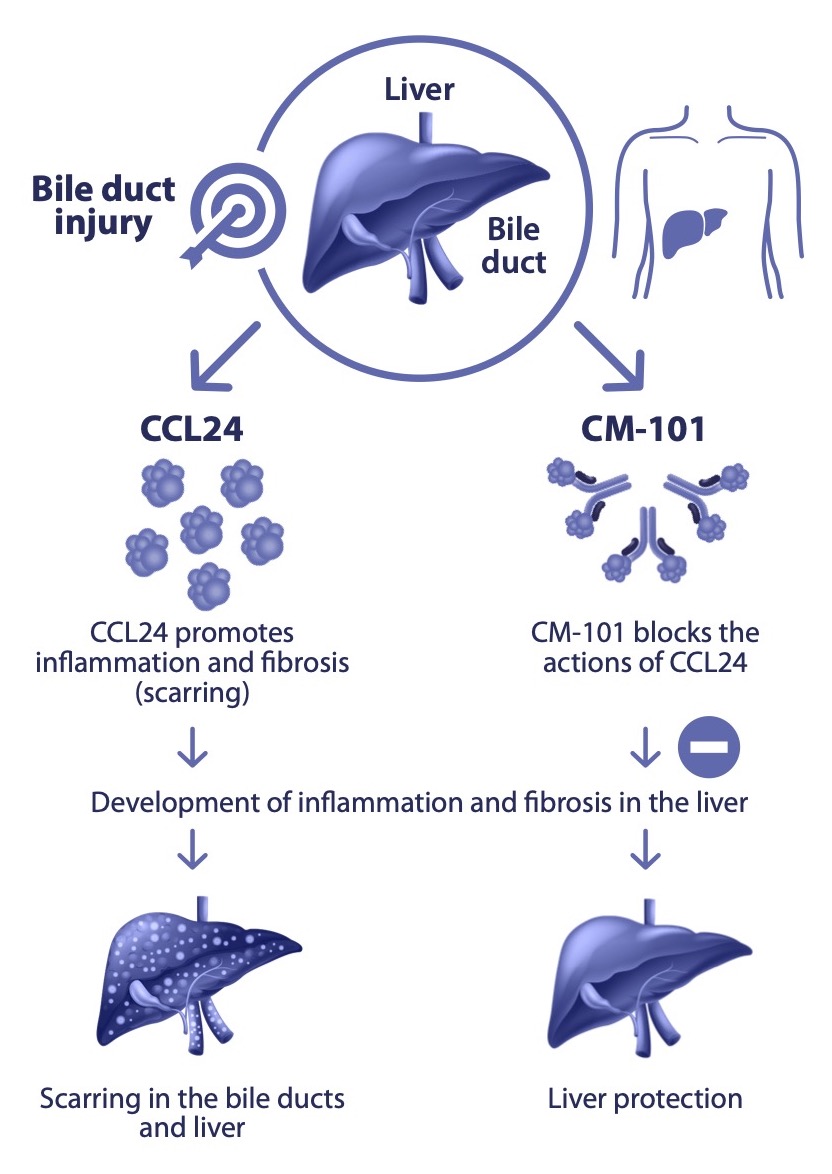
Primary Sclerosing Cholangitis (PSC) is a chronic progressive cholestatic disease of the liver characterized by inflammation and strictures of the biliary tree inside and/or outside the liver. The pathological hallmarks of PSC include injury to the integrity of the biliary ducts, retention of bile acids and intrahepatic inflammation and progressive liver fibrosis.
Chemomab is Testing the Novel Biologic Drug, CM-101, Which it has Developed for Treating PSC:
- CM-101 targets the soluble protein CCL24, which was found to play a pivotal role in promoting fibrotic and inflammatory activities in the liver through its receptor, CCR3
- Chemomab has demonstrated that CCL24 is strongly expressed in PSC patients’ liver samples (biopsies), while its levels in the blood correlate with the state of liver fibrosis
Chemomab conducted preclinical tests of CM-101 in multiple PSC animal models, and found that CM-101 was effective and significantly attenuated disease severity.
Chemomab also tested CM-101 in healthy volunteers, in patients with nonalcoholic fatty liver disease (NAFLD) and in patients with nonalcoholic steatohepatitis (NASH) and found CM-101 to be safe and well tolerated at all tested doses. In the current study, Chemomab is evaluating CM-101 for treating PSC patients. The SPRING study is testing CM-101 safety and tolerability in adult PSC patients. Other endpoints include a variety of biomarkers relevant to PSC.
This is a randomized, placebo-controlled Phase 2a study, administering 5 intravenous infusions of study drug CM-101 once every 3 weeks over 15 weeks at doses of either 10mg/kg or 20mg/kg, or placebo. There is also an optional 33-week open label extension period where all patients are eligible to receive CM-101.
Patient enrollment in the SPRING study is now completed. The company expects to report a readout of topline clinical data from the trial in midyear of 2024.
CM-101 has received Orphan drug designation for the treatment of PSC from the U.S. FDA and the European Union. It has also received Fast Track designation from the FDA. These programs are designed to help facilitate the clinical development and regulatory approval of promising new therapies for serious diseases.
For more information, visit:
NIH number: NCT04595825
To learn more about how CM-101 may interrupt the fibro-inflammatory vicious cycle believed to drive disease progression in PSC, see our Mechanism of Action (MOA) video at: MOA video
EXPANDED ACCESS POLICY
Chemomab Therapeutics Ltd. (Chemomab) is a clinical-stage biotech company focusing on the discovery and development of innovative therapeutics for patients with serious and debilitating fibrotic-inflammatory diseases with high unmet medical need. Its investigational agent CM-101 is currently being developed for fibrotic-inflammatory conditions such as primary sclerosing cholangitis and systemic sclerosis.
In general, new therapeutics must be reviewed and approved by a regulatory authority, such as the U.S. Food and Drug Administration (FDA), before they become commercially available. These regulatory determinations are based on data from clinical trials designed to evaluate whether the new therapeutic is safe and effective for patients. Investigational drug products that have not completed the clinical trial and FDA regulatory review process may, or may not, be effective as a treatment, and the use of such investigational products may cause unexpected serious side effects.
Expanded access, also called compassionate use, refers to a pathway in which patients with serious or immediately life-threatening diseases may gain access to an investigational therapy outside the context of participation in clinical trials. Wherever possible, we encourage patients to participate in clinical trials, which offer the safest and best opportunity to access an investigational product before regulatory approval. We recognize that some patients who may not be eligible to participate in our clinical trials and have exhausted all available treatment options, may seek to receive CM-101 or other investigational drugs, under an expanded access protocol, before they are approved by regulatory authorities.
Chemomab is committed to evaluation of its drug candidates through the clinical trial process. At this time, Chemomab is not accepting any requests for expanded access to any of our investigational drug candidates outside of clinical trials.
Chemomab may revise this expanded access policy at any time.
For additional information
Physicians located in the U.S. may find additional information regarding expanded access to investigational products by visiting the FDA website: Expanded Access: Information for Physicians.
Information about our ongoing Phase 2 clinical trial in primary sclerosing cholangitis is available at NCT04595825
Licensed healthcare providers who have questions regarding Chemomab’s investigational drug candidates or ongoing clinical trials may contact us at Medinfo@Chemomab.com. You can find further contact details on the Contact Us page of our website.